Custom Isolation
With advanced isolation technology and unique production process ,We can solve whatever technological problems and needs of the customers in a short time. Currently,we can offer Custom isolation and purificaton service of Phytochemical reference standards、Pure Compounds、High quality extract and Impurity met along with drug development.
1、High Quality Extract
Through the modern Isolation, separation techniques and analytical techniques, we can provide the constituents of natural product ,like those of fat soluble, alcohol soluble water soluble. so do the chemical constituent of natural product,such as the total flavonoids, total alkaloids, total triterpenoids and so on.
2、Separation and purification of the Pure compounds
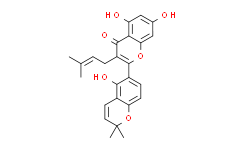
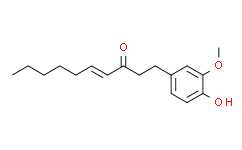
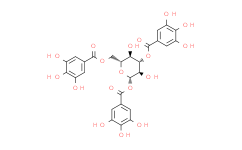
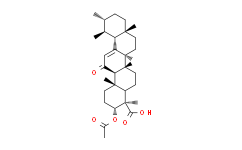
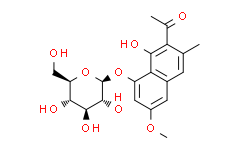
2.1 By applicable ways of separation and purification, our team can systematically separate the constituents of natural product(including the plant, fungus, marine organism, fermentation products of microorganisms) for high purity pure compounds, and provide documents of structural formula, separation process, reference literature and relevant chromatogram of each compound. The KG scale is alco acceptacble for us.
2.2 During the new drug approval process,, the structure of impurity must be confirmed or separated out when the it is more than 0.2%. ALFA Biotechnology has abundant experience in separating and identifying micro and trace impurity, so we can be a realiabe partner during ur new drug application and quality impoving of current drugs.
1、High Quality Extract
Through the modern Isolation, separation techniques and analytical techniques, we can provide the constituents of natural product ,like those of fat soluble, alcohol soluble water soluble. so do the chemical constituent of natural product,such as the total flavonoids, total alkaloids, total triterpenoids and so on.
2、Separation and purification of the Pure compounds
2.1 By applicable ways of separation and purification, our team can systematically separate the constituents of natural product(including the plant, fungus, marine organism, fermentation products of microorganisms) for high purity pure compounds, and provide documents of structural formula, separation process, reference literature and relevant chromatogram of each compound. The KG scale is alco acceptacble for us.
2.2 During the new drug approval process,, the structure of impurity must be confirmed or separated out when the it is more than 0.2%. ALFA Biotechnology has abundant experience in separating and identifying micro and trace impurity, so we can be a realiabe partner during ur new drug application and quality impoving of current drugs.